- Radiometric Dating: Methods, Uses & the Significance of Half-Life
- Navigation menu
- Radiometric dating - RationalWiki
The methods work because radioactive elements are unstable, and they are always trying to move to a more stable state.
So, they do this by giving off radiation. This process by which an unstable atomic nucleus loses energy by releasing radiation is called radioactive decay.
Radiometric Dating: Methods, Uses & the Significance of Half-Life
The thing that makes this decay process so valuable for determining the age of an object is that each radioactive isotope decays at its own fixed rate, which is expressed in terms of its half-life. So, if you know the radioactive isotope found in a substance and the isotope's half-life, you can calculate the age of the substance. So, what exactly is this thing called a half-life? Well, a simple explanation is that it is the time required for a quantity to fall to half of its starting value.
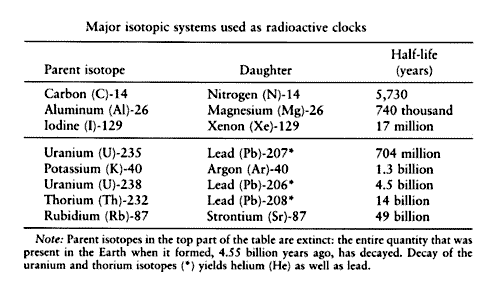
So, you might say that the 'full-life' of a radioactive isotope ends when it has given off all of its radiation and reaches a point of being non-radioactive. When the isotope is halfway to that point, it has reached its half-life. There are different methods of radiometric dating that will vary due to the type of material that is being dated. For example, uranium-lead dating can be used to find the age of a uranium-containing mineral.
It works because we know the fixed radioactive decay rates of uranium, which decays to lead, and for uranium, which decays to lead So, we start out with two isotopes of uranium that are unstable and radioactive. They release radiation until they eventually become stable isotopes of lead. These two uranium isotopes decay at different rates. In other words, they have different half-lives. The half-life of the uranium to lead is 4.
Navigation menu
The uranium to lead decay series is marked by a half-life of million years. These differing rates of decay help make uranium-lead dating one of the most reliable methods of radiometric dating because they provide two different decay clocks. This provides a built-in cross-check to more accurately determine the age of the sample.
Uranium is not the only isotope that can be used to date rocks; we do see additional methods of radiometric dating based on the decay of different isotopes. For example, with potassium-argon dating , we can tell the age of materials that contain potassium because we know that potassium decays into argon with a half-life of 1. With rubidium-strontium dating , we see that rubidium decays into strontium with a half-life of 50 billion years.
By anyone's standards, 50 billion years is a long time. In fact, this form of dating has been used to date the age of rocks brought back to Earth from the moon. So, we see there are a number of different methods for dating rocks and other non-living things, but what if our sample is organic in nature? For example, how do we know that the Iceman, whose frozen body was chipped out of glacial ice in , is 5, years old? Well, we know this because samples of his bones and hair and even his grass boots and leather belongings were subjected to radiocarbon dating.
Radiocarbon dating , also known as carbon dating or simply carbon dating, is a method used to determine the age of organic material by measuring the radioactivity of its carbon content. So, radiocarbon dating can be used to find the age of things that were once alive, like the Iceman.
- dating a kenyan woman in america.
- dreams about dating your ex boyfriend.
- dating matchmaking vancouver!
- Description.
- speed dating cruise chester.
- You must create an account to continue watching?
- Radiometric Dating.
And this would also include things like trees and plants, which give us paper and cloth. So, radiocarbon dating is also useful for determining the age of relics, such the Dead Sea Scrolls and the Shroud of Turin. With radiocarbon dating, the amount of the radioactive isotope carbon is measured. Compared to some of the other radioactive isotopes we have discussed, carbon's half-life of 5, years is considerably shorter, as it decays into nitrogen Carbon is continually being created in the atmosphere due to the action of cosmic rays on nitrogen in the air.
Carbon combines with oxygen to create carbon dioxide.
Because plants use carbon dioxide for photosynthesis, this isotope ends up inside the plant, and because animals eat plants, they get some as well. When a plant or an animal dies, it stops taking in carbon The existing carbon within the organism starts to decay back into nitrogen, and this starts our clock for radiocarbon dating. A scientist can take a sample of an organic material when it is discovered and evaluate the proportion of carbon left in the relic to determine its age. Radiometric dating is a method used to date rocks and other objects based on the known decay rate of radioactive isotopes.
The decay rate is referring to radioactive decay , which is the process by which an unstable atomic nucleus loses energy by releasing radiation. Each radioactive isotope decays at its own fixed rate, which is expressed in terms of its half-life or, in other words, the time required for a quantity to fall to half of its starting value. There are different methods of radiometric dating. Uranium-lead dating can be used to find the age of a uranium-containing mineral.
Uranium decays to lead, and uranium decays to lead The two uranium isotopes decay at different rates, and this helps make uranium-lead dating one of the most reliable methods because it provides a built-in cross-check. Additional methods of radiometric dating, such as potassium-argon dating and rubidium-strontium dating , exist based on the decay of those isotopes. Radiocarbon dating is a method used to determine the age of organic material by measuring the radioactivity of its carbon content. With radiocarbon dating, we see that carbon decays to nitrogen and has a half-life of 5, years.
To unlock this lesson you must be a Study. Did you know… We have over college courses that prepare you to earn credit by exam that is accepted by over 1, colleges and universities. You can test out of the first two years of college and save thousands off your degree. Anyone can earn credit-by-exam regardless of age or education level.
To learn more, visit our Earning Credit Page. Not sure what college you want to attend yet? The videos on Study. Students in online learning conditions performed better than those receiving face-to-face instruction. Explore over 4, video courses. Find a degree that fits your goals. Learn about half-life and how it is used in different dating methods, such as uranium-lead dating and radiocarbon dating, in this video lesson.
Try it risk-free for 30 days. An error occurred trying to load this video. Try refreshing the page, or contact customer support. Register to view this lesson Are you a student or a teacher? I am a student I am a teacher. What teachers are saying about Study. Conditions of Fossil Preservation: Are you still watching? Your next lesson will play in 10 seconds. Add to Add to Add to. Want to watch this again later? What is Radioactive Dating? Principles of Radiometric Dating. Relative Dating with Fossils: Index Fossils as Indicators of Time. Carbon is a very special element.
In combination with hydrogen it forms a component of all organic compounds and is therefore fundamental to life.
Radiometric dating - RationalWiki
Libby of the University of Chicago predicted the existence of carbon before it was actually detected and formulated a hypothesis that radiocarbon might exist in living matter. Willard Libby and his colleague Ernest Anderson showed that methane collected from sewage works had measurable radiocarbon activity whereas methane produced from petroleum did not. Perseverance over three years of secret research to develop the radiocarbon method came into fruition and in Libby received the Nobel Prize for chemistry for turning his vision into an invaluable tool. Carbon has three naturally occurring isotopes , with atoms of the same atomic number but different atomic weights.
They are 12 C, 13 C and 14 C. C being the symbol for carbon and the isotopes having atomic weights 12, 13 and The three isotopes don't occur equally either, The radiocarbon dating method is based on the rate of decay of the radioactive or unstable 14 C which is formed in the upper atmosphere through the effect of cosmic ray neutrons upon nitrogen The reaction is as follows: After formation the three carbon isotopes combine with oxygen to form carbon dioxide. The carbon dioxide mixes throughout the atmosphere, dissolves in the oceans, and via photosynthesis enters the food chain to become part of all plants and animals.
In principle the uptake rate of 14 C by animals is in equilibrium with the atmosphere. As soon as a plant or animal dies, they stop the metabolic function of carbon uptake and with no replenishment of radioactive carbon, the amount of 14 C in their tissues starts to reduce as the 14 C atoms decay.