- BBC - GCSE Bitesize: Radioactive dating
- Counting carbon 14 atoms in a bygone object to find its age
- Carbon dating
- Radiocarbon Dating
Aluminum containers with screw caps are safe, but it is still best to consult the radiocarbon laboratory for the best containers of carbon dating samples. It is recommended that archaeologists, or any client in general, ask the laboratory if results have systematic or random errors. They should also ask details about the calibration used for conversion of BP years to calendar years. Clarify the costs involved in radiocarbon dating of samples. Some labs charge more for samples that they do not regularly process.
Radiocarbon dating takes time, and laboratories often have waiting lists so this factor must be considered. The carbon dating process is destructive, and labs usually advise their clients with regard to sample identification or labelling.
BBC - GCSE Bitesize: Radioactive dating
Communication with clients also gives labs an idea of the possible types of contaminants in the excavation site. Knowing the type of contaminants also give radiocarbon scientists an idea on the pretreatment methods needed to be done before starting carbon dating. Labs ask clients on the expected age of the radiocarbon dating samples submitted to make sure that cross-contamination is avoided during sample processing and that no sample of substantial age more than 10, years must follow modern ones.
Labs also want to avoid processing carbon dating samples that will yield large calendar ranges. Radiocarbon dating results have insignificant value as in the case when the calibration curve is effectively flat and all calendar events in the period will produce about the same radiocarbon age. In either of the cases, it is still worthwhile to carefully consider why the radiocarbon dating results were deemed unacceptable.
Rescue archaeology involves the survey and potential excavation of sites that are to undergo some form of construction or development in order to recover any valuable finds that are uncovered and prevent their destruction. The impending developments leave little time for archaeologists to undertake their work and creates a time-pressured environment with stakeholders eager for them to finish as soon as possible. In such cases where potentially valuable finds are discovered, fast and high-quality radiocarbon dating results can be crucial in determining whether a site warrants further excavation or can be handed back to the developers.
In particular, time-sensitive projects like rescue archaeology , waiting months for test results while construction is halted is not viable and can be a financial burden.
Archaeologists need radiocarbon dating laboratories that can cater to their specific project requirements and deadlines. Sheridan Bowman, Radiocarbon Dating: Interpreting the Past , University of California Press. Accelerator Mass Spectrometry AMS dating involves accelerating ions to extraordinarily high kinetic energies followed by mass analysis. Carbon is a radioactive isotope of carbon it has two extra neutrons in its nucleus making it unstable. South Tyrol Museum of Archaeology.
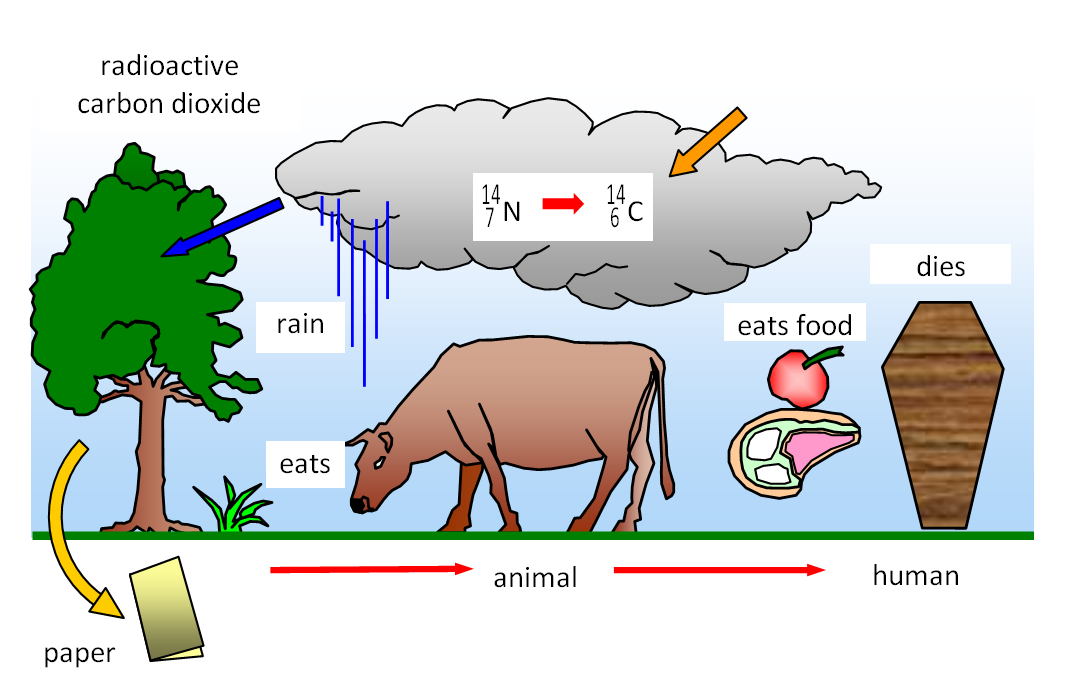
There's a small amount of radioactive carbon in all living organisms. When they die no new carbon is taken in by the dead organism.
Counting carbon 14 atoms in a bygone object to find its age
The carbon it contained at the time of death decays over a long period of time. By measuring the amount of carbon left in dead organic material the approximate time since it died can be worked out. Radiocarbon Dating is the process of determining the age of a sample by examining the amount of 14 C remaining against the known half-life, 5, years.
The reason this process works is because when organisms are alive they are constantly replenishing their 14 C supply through respiration, providing them with a constant amount of the isotope. However, when an organism ceases to exist, it no longer takes in carbon from its environment and the unstable 14 C isotope begins to decay.
Carbon dating
From this science, we are able to approximate the date at which the organism were living on Earth. Radiocarbon dating is used in many fields to learn information about the past conditions of organisms and the environments present on Earth. Radiocarbon dating usually referred to simply as carbon dating is a radiometric dating method.
It uses the naturally occurring radioisotope carbon 14C to estimate the age of carbon-bearing materials up to about 58, to 62, years old. Carbon has two stable, nonradioactive isotopes: There are also trace amounts of the unstable radioisotope carbon 14 C on Earth. Carbon has a relatively short half-life of 5, years, meaning that the fraction of carbon in a sample is halved over the course of 5, years due to radioactive decay to nitrogen The carbon isotope would vanish from Earth's atmosphere in less than a million years were it not for the constant influx of cosmic rays interacting with molecules of nitrogen N 2 and single nitrogen atoms N in the stratosphere.
Both processes of formation and decay of carbon are shown in Figure 1.
Radiocarbon Dating
Diagram of the formation of carbon forward , the decay of carbon reverse. Carbon is constantly be generated in the atmosphere and cycled through the carbon and nitrogen cycles. Once an organism is decoupled from these cycles i. When plants fix atmospheric carbon dioxide CO 2 into organic compounds during photosynthesis, the resulting fraction of the isotope 14 C in the plant tissue will match the fraction of the isotope in the atmosphere and biosphere since they are coupled.