- Radioactive Dating | media-aid.com
- Radiometric Dating
- Dating Fossils – How Are Fossils Dated?
- Radiometric Dating: Methods, Uses & the Significance of Half-Life
Radiocarbon dating was used to identify a forged painting based upon the concentrations of carbon detected on the canvas within the atmosphere at the time that the picture was painted. So, to sum this all up, radioactive dating is the process scientists use to conclude the ages of substances dating back several to many years ago by using the isotopes of elements and their half-lives. An isotope is a variation of an element based upon the number of neutrons.
The disintegration of the neutrons within the atom of the element's nucleus is what scientists call radioactivity. An isotope disintegrates at a constant rate called the half-life , or the time it takes for half the atoms of a sample to decay. The half-life can also be termed an atomic clock. By counting the number of half-lives and the percentages remaining of parent and daughter isotopes, scientists are able to determine what they call the absolute age of a discovery.
- RADIOMETRIC DATING - Definition and synonyms of radiometric dating in the English dictionary.
- dating sites in boston ma.
- Navigation menu.
- istanbul dating service.
- Translation of «radiometric dating» into 25 languages.
- Relative Dating.
- new york dating online.
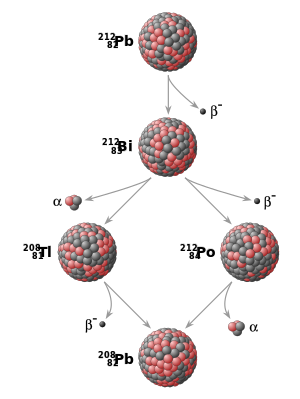
Carbon is a specific isotope used in dating materials that were once living. Other common isotopes used in radioactive dating are uranium, potassium, and iodine.
Radioactive Dating | media-aid.com
To unlock this lesson you must be a Study. Did you know… We have over college courses that prepare you to earn credit by exam that is accepted by over 1, colleges and universities. You can test out of the first two years of college and save thousands off your degree. Anyone can earn credit-by-exam regardless of age or education level. To learn more, visit our Earning Credit Page. Not sure what college you want to attend yet? The videos on Study. Students in online learning conditions performed better than those receiving face-to-face instruction.
Explore over 4, video courses.
Radiometric Dating
Find a degree that fits your goals. What is Radioactive Dating? Try it risk-free for 30 days. An error occurred trying to load this video. Try refreshing the page, or contact customer support.
Dating Fossils – How Are Fossils Dated?
Register to view this lesson Are you a student or a teacher? I am a student I am a teacher. What teachers are saying about Study. Are you still watching? Your next lesson will play in 10 seconds. Add to Add to Add to. Want to watch this again later? Principles of Radiometric Dating. What is Carbon Dating? What is Relative Age? What is Relative Dating?
Absolute Time in Geology. Relative Dating with Fossils: Index Fossils as Indicators of Time. Methods of Geological Dating: Numerical and Relative Dating. Major Eons, Eras, Periods and Epochs. Prentice Hall Earth Science: Holt McDougal Earth Science: ScienceFusion Matter and Energy: Discover how scientists determine the age of fossils, rocks, and other geologic phenomena by using the known half-lives of isotopes within each specimen, a technique known as radioactive dating.
Radioactive Dating Ever wonder how scientists concluded the age of the earth to be about 4. Radioactivity Defined Elements occur naturally in the earth, and they can tell us a lot about its past. The Half-Life Isotopes decay at a constant rate known as the half-life. A method for determining the age of an object based on the concentration of a particular radioactive isotope contained within it and the half-life of that isotope.
Radiometric Dating: Methods, Uses & the Significance of Half-Life
A method for determining the age of an object based on the concentration of a particular radioactive isotope contained within it. The amount of the isotope in the object is compared to the amount of the isotope's decay products. The object's approximate age can then be figured out using the known rate of decay of the isotope. Radiocarbon dating is one kind of radiometric dating, used for determining the age of organic remains that are less than 50, years old.
For inorganic matter and for older materials, isotopes of other elements, such as potassium, uranium, and strontium, are used. Dating rocks by the known rate of decay of radioactive elements that they contain. References in periodicals archive? He does show a misunderstanding of Christian faith when he writes about radiometric dating of the earth.
Since the discovery of radioactive dating, there have been several improvements in the equipment used to measure radioactive residuals in samples.
For example, with the invention of accelerator mass spectometry, scientists have been able to date samples very accurately. See also Radioactive decay. The discovery of the radioactive properties of uranium in by Henri Becquerel subsequently revolutionized the way scientists measured the age of artifacts and supported the theory that the earth was considerably older than what some scientists believed.
There are several methods of determining the actual or relative age of the earth's crust: Although the half-life of rubidium is even longer than uranium 49 billion years or 10 times the age of the earth , it is useful because it can be found in almost all igneous rocks. In , a radioactive dating method for determining the age of organic materials, was developed by Willard Frank Libby , who received the Nobel Prize in Chemistry in for his radiocarbon research.
All living plants and animals contain carbon , and while most of the total carbon is carbon, a very small amount of the total carbon is radioactive carbon Libby, and his team of researchers, measured the amount of carbon in a piece of acacia wood from an Egyptian tomb dating b. Scientists are able to study recent climactic events by measuring the amount of a specific radioactive nuclide that is known to have attached itself to certain particles that have been incorporated into the earth's surface.
For example, during the s, when many above-ground tests of nuclear weapons occurred, the earth was littered by cesium half-life of Radon decays to polonium, which attaches to particles in the atmosphere and is consequently rained out—falling into and traveling through streams, rivers , and lakes. Radioactive dating has proved to be an invaluable tool and has been used in many scientific fields, including geology , archeology, paleoclimatology, atmospheric science, oceanography , hydrology , and biomedicine. This method of dating has also been used to study artifacts that have received a great deal of public attention, such as the Shroud of Turin , the Dead Sea Scrolls , Egyptian tombs, and Stonehenge.
Radioactive dating is a method of determining the approximate age of an old object by measuring the amount of a known radioactive element it contains. Rocks as well as fossil plants and animals can be dated by this process. It has given paleontologists a person specializing in the study of fossils as well as geologists a person specializing in the study of the origin, history, and structure of Earth a powerful way of dating ancient objects. Until the discovery of radioactive dating , scientists had no way of approximating how old any part of Earth was.
Once the principle behind this method was discovered, however, it became possible to gather reliable information about the age of Earth and its rocks and fossils. Radioactive dating was not possible until , when the radioactive properties of uranium a radioactive metallic element were discovered by French physicist a person specializing in the study of energy and matter , Antoine Henri Becquerel — When a substance is described as radioactive, it means that at the subatomic relating to parts of an atom level, some parts of it are unstable. When a substance is described as unstable, it means that it has a tendency to break down or decay.
During this decay, one substance actually changes into another and radiation is released. As long ago as , the American chemist Bertram B. Boltwood — suggested that knowledge of radioactivity might be used to determine the age of Earth's crust. He suggested this because he knew that the end product of the decay of uranium was a form of lead.
Since each radioactive element decays at a known rate, it can be thought of as a ticking clock. Boltwood explained that by studying a rock containing uranium, its age could be determined by measuring its amounts of uranium and lead. The more lead the rock contained, the older it was.
Although this was a major breakthrough, Boltwood's dating method made it possible to date only the oldest rocks. This is because uranium decayed or changed into lead at such a slow rate that it was not reliable for measuring the age of rocks that were younger than 10,, years old. Another drawback was that uranium is not found in every rock. A later method that used rubidium which changes into strontium proved more useful because it is found in nearly all rocks, although it still was not useful for younger specimens.